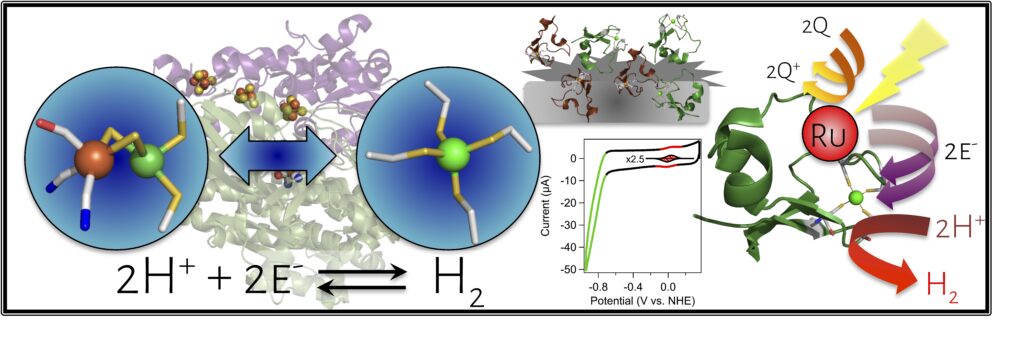
The [NiFe] hydrogenase enzymes are exemplary catalysts, containing well-structured primary, secondary, tertiary, and quaternary coordination environments that enable efficient, fast, and reversible turnover. On the other hand, given sufficient driving force, many synthetic compounds will evolve hydrogen gas in the presence of protons, though they are typically inert towards hydrogen oxidation. What makes hydrogenases, then, so special? Are the many evolved layers of complexity necessary for activity? What molecular-level factors control catalytic rates, overpotential, and bias?
We are addressing these questions by reconstructing hydrogenases (and other enzymes) within repurposed metalloprotein scaffolds. We have developed nickel-substituted rubredoxin as a protein-based model of the [NiFe] hydrogenase that reproduces the primary coordination sphere of the native enzyme, demonstrating high levels of catalytic activity towards H2 evolution. We are now applying an array of spectroscopic and analytical techniques to establish the catalytic mechanism and elucidate the role of the secondary coordination environment and protein dynamics in catalysis. In addition, the robustness of the nickel rubredoxin system makes it well-suited for large-scale hydrogen production applications, and we are actively pursuing opportunities for extending our impact beyond the bench.
