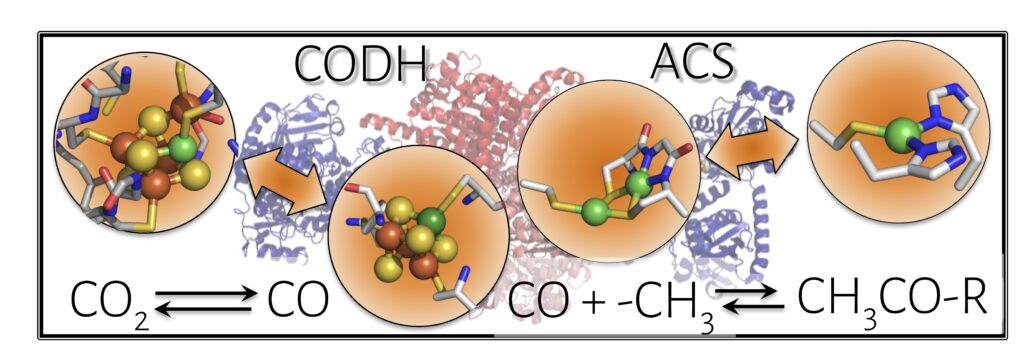
Nickel-containing metalloenzymes are central to the primary metabolic processes of ancient organisms and implicated for chemoautotrophic origins of life, as they catalyze hydrogen oxidation, CO2 fixation, methane generation, and carbon-carbon bond formation to generate essential cellular building blocks. These reactions are also highly valuable from the standpoint of energy and environment. However, due to substantial challenges associated with studying these native enzymes, much remains unknown about the basic catalytic mechanisms, hindering efforts to harness this chemistry for anthropogenic purposes.
We are developing structural, functional, and mechanistic models of reductive nickel enzymes such as carbon monoxide dehydrogenase and acetyl coenzyme A synthase within robust protein scaffolds. By combining functional studies of our model proteins with diverse spectroscopic techniques and computational investigations, we can obtain a comprehensive understanding of how the electronic and geometric structures dictate reactivity in each system. Reconstructing functional metalloenzymes “from the ground up” offers direct insight into the fundamental chemical principles driving the natural systems. Looking forward, we hope to apply these principles towards engineering effective systems for energy conversion reactions while learning about fundamental chemical transformations that may underlie the evolution of prebiotic processes into early life.
