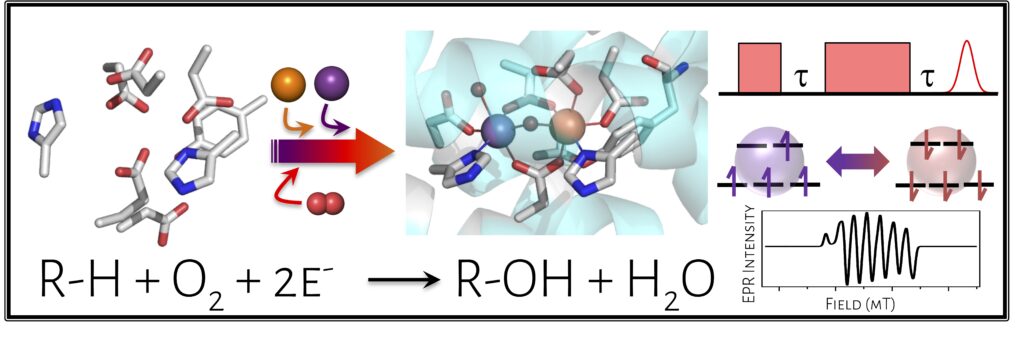
Non-heme diiron (Fe/Fe) oxidase enzymes are ubiquitous and carry out challenging chemical transformations, from deformylation to radical generation to aliphatic hydroxylation, using only oxygen as the oxidant. A related class of enzymes have been identified relatively recently to carry out analogous reactions using a heterobimetallic manganese-iron (Mn/Fe) cofactor. These Mn/Fe proteins are prevalent in extremophiles and pathogens; notably, a Mn/Fe protein called R2lox (ribonucleotide reductase subunit 2-like ligand-binding oxidase) has been shown to be upregulated in virulent strains of Mycobacterium tuberculosis, suggesting a role in pathogenicity. However, little is known about these new classes of proteins, including their in vivo function or in vitro reactivity.
We are interested in understanding the chemical capacity of this new Mn/Fe cofactor and the molecular-level determinants controlling metal binding, oxygen activation, C-H bond cleavage, and downstream reactivity. In these studies, we employ a range of spectroscopic techniques, including pulsed EPR, X-ray absorption, and resonance Raman spectroscopy. Additionally, we are pursuing microbiological studies to uncover the functional role of R2lox across diverse organisms. This project will help us understand when and why nature may select for the non-canonical Mn/Fe cofactor in R2lox and related proteins.
